Abstract
Introduction: Current Hodgkin lymphoma (HL) therapies result in 80-90% progression-free survival (PFS). However, about 10-20% of patients with advanced disease relapse. The standard treatment for primary progressive or relapsed disease is salvage chemotherapy. For patients responding to this treatment, high-dose chemotherapy followed by autologous stem cell transplantation (ASCT) is recommended. In a recently published study, an addition of post-transplant therapy has improved PFS. However, as this approach is associated with additional toxicity and increased financial burden, precise identification of patients who could benefit from this strategy is crucial. Our study provides outcome data of an unselected group of HL patients and suggests a model identifying individuals at high risk of disease progression in whom post-ASCT therapy could be advantageous.
Methods: This retrospective study included 92 HL patients with a median age of 31years (18-66) at diagnosis and 36 years (20-67) at time of transplant, who underwent ASCT at Rambam and continued follow-up until 08/01/2017.
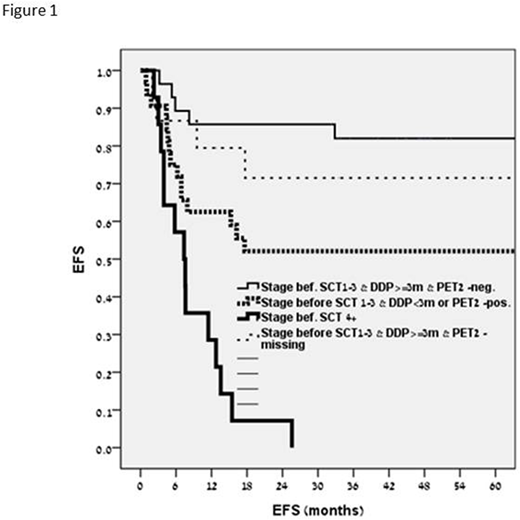
Results: At a median follow-up of 48 months (0-195) for patients with an uneventful course and 6 months (1-33) for those who progressed, the relapse rate was 41%. The Kaplan Meyer plot for event-free survival (EFS) is presented in Fig 1. In group 1, including patients with negative PET-2 during first-line therapy, who had stage I-III HL at relapse, that occurred at ≥3 months from the end of therapy (n=43), a 5-year EFS was 0.82. In group 2, including patients with the parameters similar to those in group 1, but in whom PET-2 had not been performed (n=16), a 5-year EFS was 0.71. In group 3, incorporating patients with stage I-III HL at relapse that occurred at <3 months from the end of therapy or those with positive PET-2 (n=15) a 5-year EFS was 0.52. Group 4, comprising patients with stage IV disease at relapse (n=18), had a 5-year EFS of 0. In a bivariate analysis, HL stage IV at relapse (HR 2.56, 95% CI 1.06-6.15; p=0.036), positive PET-2 during first-line therapy (HR 2.41, 95%CI 1.1-5; p<0.02) and PET positivity post-salvage therapy (HR 2.39, 95%CI 1.24- 4.6; p=0.009) were associated with increased risk of disease progression after ASCT. The factors associated with improved prognosis were: age ≥40 years at transplant (HR 0.27, 95% CI 0.11-0.66; p=0.004) and relapse at ≥6 months from the end of therapy (HR 0.31 95% CI 0.11-0.92; p=0.035). In multivariate analysis, disease progression was associated with HL stage IV at relapse (HR 11.8, 95% CI of 4-34; p=0.000), PET-2 positivity or HL progression at <3 months from the end of therapy (HR 3.5, 95%CI 1.3-9.7; p=0.015).
Conclusions: The current study suggests a 3-risk-factor model for prediction of post-ASCT HL progression, including stage IV disease at relapse, PET-2 positivity at first-line treatment or HL progression at <3 months from the end of therapy. Upon verification of our findings in an independent cohort, the suggested model could be employed in decision making regarding preemptive post-ASCT use of new therapeutic approaches, including biological and checkpoint inhibitor therapies, in HL patients who are at an increased relapse risk.
Zuckerman:Cellect Biotherapeutics Ltd: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal